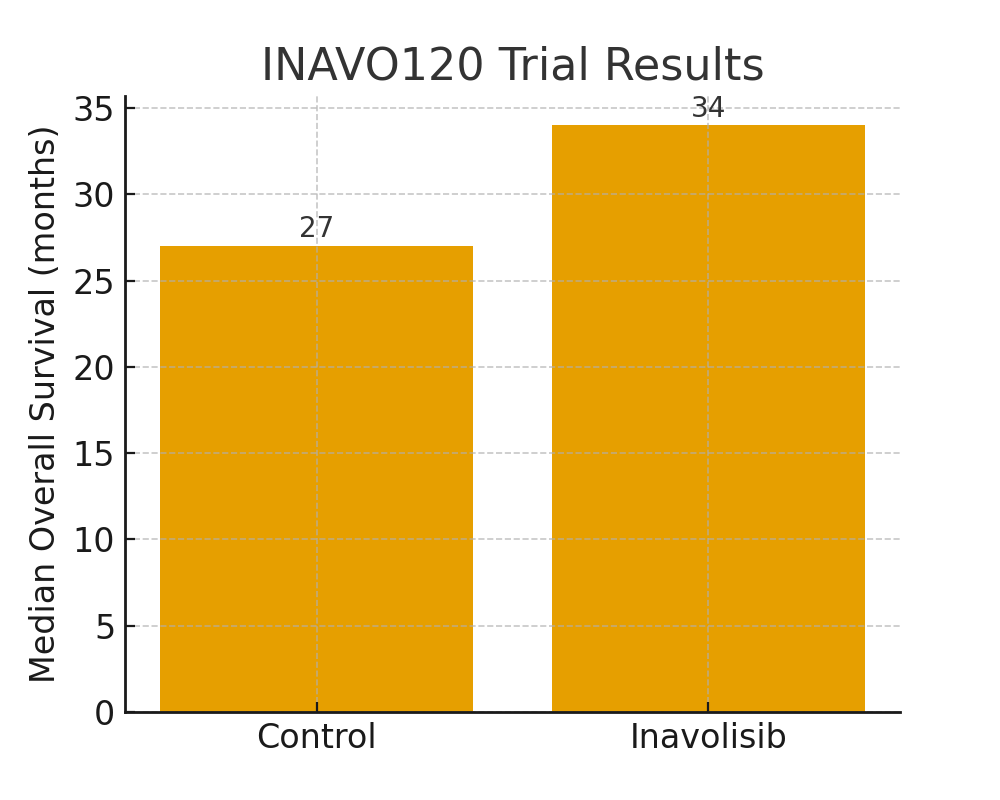
Breast cancer research is advancing rapidly, and 2025 marks a pivotal year for several groundbreaking therapies. Patients, oncologists, and researchers are seeing hope emerge not just in extended survival rates, but also in new classes of drugs that bring treatment options tailored to specific tumor biology. Below, we explore the most promising treatments, trials, and technologies shaping the future of breast cancer care.
2. Datopotamab Deruxtecan (Datroway) – A New Antibody-Drug Conjugate
What it is: Datopotamab Deruxtecan (Datroway) is a Trop-2–directed antibody-drug conjugate (ADC). It delivers a chemotherapy payload directly to tumor cells while sparing most healthy tissue.
Regulatory status:
- U.S. approval:
- EU approval:
“ADCs are changing the game. With Datroway, patients who’ve gone through multiple lines of therapy are seeing responses we wouldn’t have expected just five years ago.”
— Dr. Elena Martinez, UCSF Oncologist
Why it matters: This drug expands the toolkit for patients whose disease has become resistant to both endocrine therapy and chemotherapy.
3. Next-Generation SERDs: Camizestrant & Giredestrant
Camizestrant (AstraZeneca)
Phase III trials combining it with CDK4/6 inhibitors show improved progression-free survival over current standards.
Giredestrant (GDC-9545, Roche)
An oral SERD with FDA Fast Track status for ER⁺/HER2⁻ breast cancer. Early data suggest superior efficacy and tolerability compared with fulvestrant.
Why they matter: For decades, fulvestrant was the backbone of ER-targeted therapy, but it required injections and had limited efficacy. These oral SERDs represent a paradigm shift toward more convenient and effective hormone therapies.
4. Innovative Clinical Trials & Novel Approaches
- Vepdegestrant (PROTAC therapy): A next-generation estrogen receptor degrader that uses protein degradation technology — promising stronger activity than fulvestrant.
- ARX788 (ADC): In Phase II trials at UCSF for HER2-low cancers, addressing a group of patients historically underrepresented in HER2 therapies.
- Photodynamic “smart bombs”: Light-activated therapies that eradicate metastatic tumors in preclinical models with minimal side effects; human trials may follow.
- Eftilagimod Alpha (“Efti”): An immune-activating LAG-3 fusion protein in Phase II trials — part of the wave of immunotherapies beyond PD-1/PD-L1 inhibitors.
- Theranostics (Lutetium-177 NeoBOMB1): A dual diagnostic-therapeutic approach under Phase I/IIa investigation for GRPR-positive tumors.
5. Preventive Frontier: Breast Cancer Vaccine
What it does: Targets α-lactalbumin, a protein present in many cases of triple-negative breast cancer (TNBC). Phase I trials showed strong immune activation without major safety concerns.
Next steps: Phase II trials are scheduled for 2026, with potential expansion beyond TNBC.
Why it matters: If successful, this vaccine could represent a shift from treatment to prevention, especially for aggressive subtypes with limited therapeutic options.
6. Shifting Toward Personalized Treatment & Early Detection
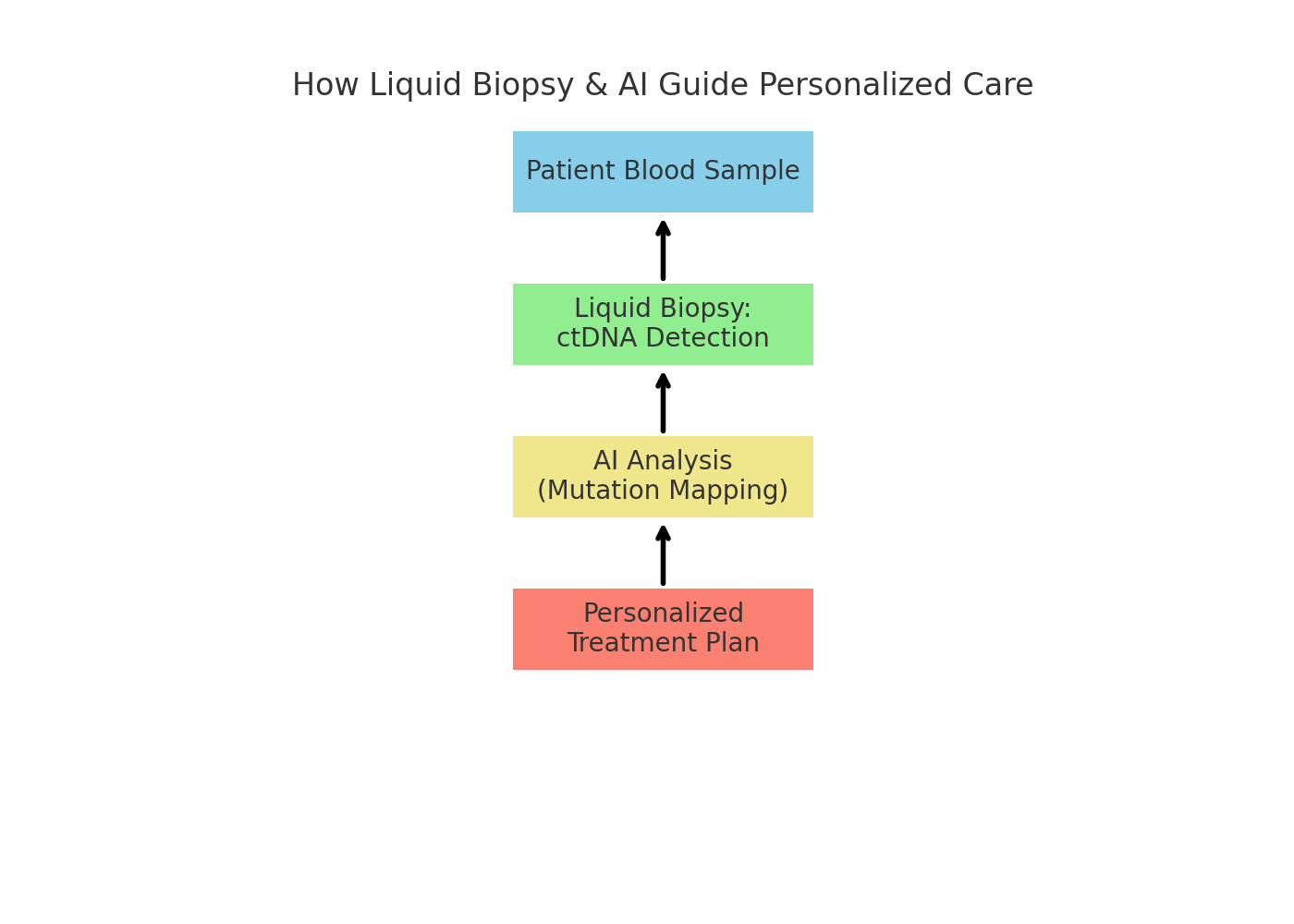
Liquid biopsies: Allow detection of circulating tumor DNA in blood, helping oncologists track mutations and treatment resistance in real time.
AI-guided treatment plans: Early research shows AI systems built on NCCN guidelines provide accurate, personalized treatment suggestions, potentially reducing disparities in care across hospitals.
“AI won’t replace oncologists, but it will help us make better decisions faster — especially in community hospitals where clinical trial access is limited.”
— Dr. Michael Chen, Mayo Clinic
Clear Takeaways for Patients & Families
- Precision is the future: Therapies like inavolisib and SERDs highlight the importance of genetic and molecular testing.
- Hope for the hardest cases: ADCs and vaccines are expanding the arsenal for patients with limited options.
- Trials matter: Many of the most promising treatments are still in research phases — clinical trial participation is critical.
- Technology + biology: AI and liquid biopsies are reshaping how quickly doctors can adjust treatment plans.